
Secure by Design
Catching Critical Vulnerabilities Before They Become Recalls The medical device industry has recently witnessed one of the most severe cybersecurity failures in its history. A
" " indicates required fields

Catching Critical Vulnerabilities Before They Become Recalls The medical device industry has recently witnessed one of the most severe cybersecurity failures in its history. A

The acquisition delivers faster compliance, stronger supply-chain security, and unparalleled MedTech expertise, while positioning C2A Security for accelerated global growth Featured Assets C2A Security Acquires
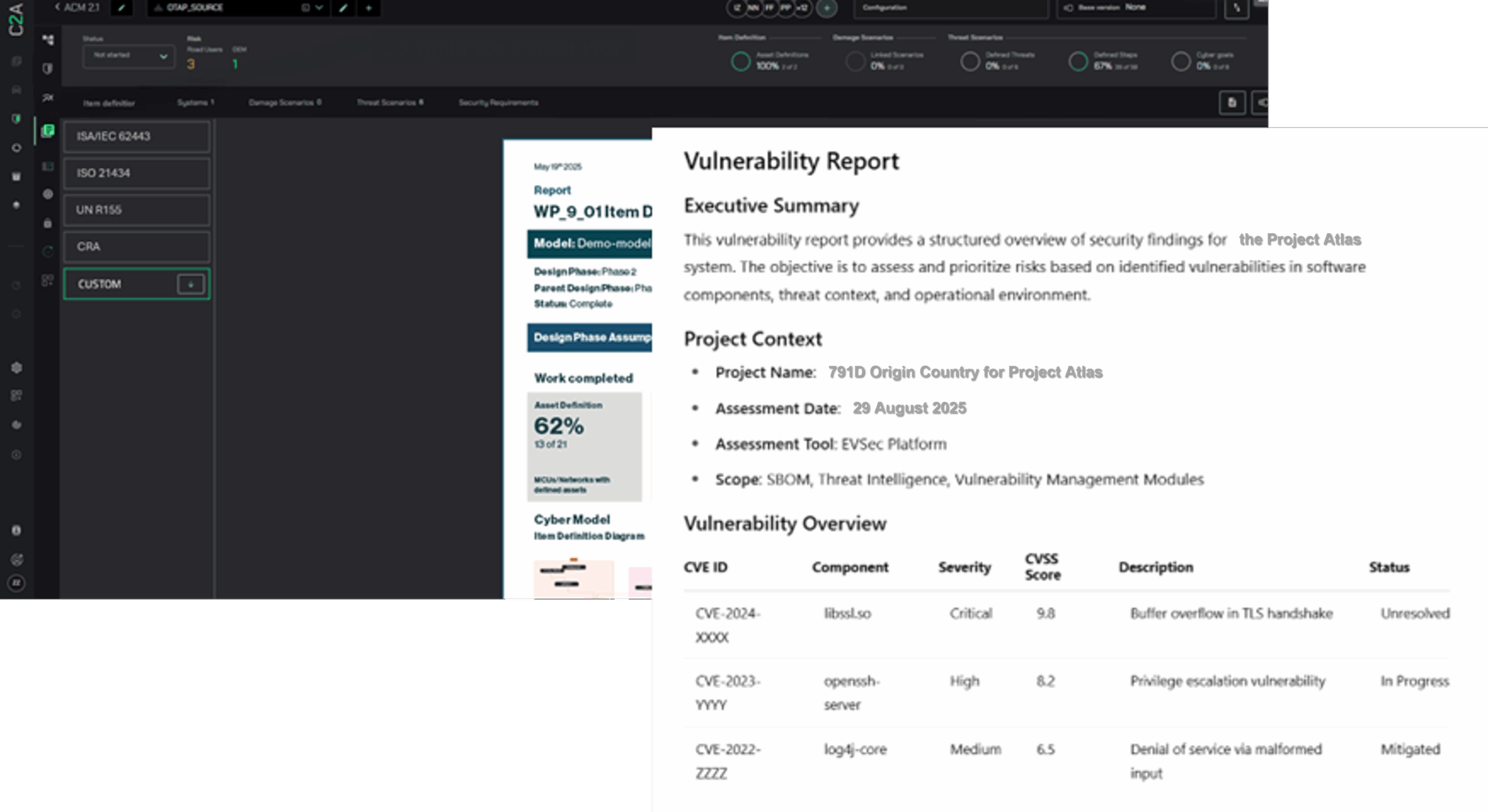
Rule 791D has officially arrived. Earlier this year, we covered the proposed ban on connected vehicle software and hardware manufactured in China and Russia. The

The partnership will leverage EVSec platform with HARMAN’s Automotive Engineering Services capabilities to automate compliance and regulatory monitoring with US DoC 791D Rule Jerusalem, Israel,

The new capability is designed to give organizations the tools and automation they need to meet regulatory requirements quickly, confidently, and without slowing innovation Jerusalem,

A seasoned sales executive in the cybersecurity sector, John will lead the company’s revenue strategy as it enters a new growth phase Jerusalem, Israel, September

Why The New FDA Update Matters Right Now for MDMs The FDA’s Final Cybersecurity Guidance of June 2025 introduces significant new requirements under the FD&C
In early 2025, security researchers uncovered a critical vulnerability in the Contec CMS8000 patient monitor system – an affordable, widely deployed device in hospitals and

Integration across the company’s product lifecycle to help meet evolving regulatory standards and improve security Jerusalem, Israel, May 20, 2025 – C2A Security today announced

CRO
C2A Security

VP and GM, Medical Technology
C2A Security
Ken Zalevsky brings over 20 years of medical device cybersecurity experience to his role at C2A Security, where he serves as VP and GM, Medical Technology, following the acquisition of Vigilant Ops in October 2025. A former Bayer executive, Ken founded Vigilant Ops in 2019 after witnessing the consequences of inadequate technical preparation in the healthcare industry. He is an active contributor to CISA’s SBOM working groups and a frequent speaker on software supply chain security. Ken’s mission: transform SBOM from a compliance checkbox into operational intelligence that keeps patients safe while streamlining regulatory processes.