" " indicates required fields
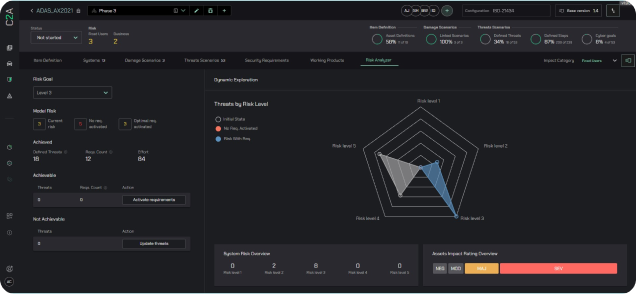
Establish a robust analytical infrastructure to assess project risks systematically, promoting dynamic risk management, throughout the product lifecycle, and using risk information for automation.
Streamlined Compliance
Dynamic and adjustable reports for scalable compliance with regulations, streamlining the compliance process.
Advanced Risk Analysis, Automated Policy Enforcement
Leverage data-driven insights for risk management, development, and operations, enabling proactive and optimized decision-making with new development projects, vulnerabilities, and security events.
Accelerated Product Delivery with Enhanced Efficiency
Expedite security assessments and product security workflows, resulting in faster and more cost-efficient release cycles.
Utilize Threat Modeling for Product Security Automation
Use EVSec’s patented analytic threat modeling approach to drive automation throughout the product development and operations lifecycle.


CRO
C2A Security

VP and GM, Medical Technology
C2A Security
Ken Zalevsky brings over 20 years of medical device cybersecurity experience to his role at C2A Security, where he serves as VP and GM, Medical Technology, following the acquisition of Vigilant Ops in October 2025. A former Bayer executive, Ken founded Vigilant Ops in 2019 after witnessing the consequences of inadequate technical preparation in the healthcare industry. He is an active contributor to CISA’s SBOM working groups and a frequent speaker on software supply chain security. Ken’s mission: transform SBOM from a compliance checkbox into operational intelligence that keeps patients safe while streamlining regulatory processes.